Abstract
Background: Salvage autologous stem cell transplantations (ASCT) in the setting of relapsed multiple myeloma have historically been an important therapeutic option. There have not been any comparative studies looking at this approach in the era of novel therapeutic agents such as monoclonal antibodies and emerging immunotherapies. It is important to have a real world benchmark when understanding the landscape of potential treatments in this space. Maintenance therapy post frontline autologous stem cell transplantation has become standard of care due to the important improvement in progression free survival (PFS) as well as overall survival (OS). However, little is known about the impact of maintenance post-salvage transplant. The objectives of this study are to define PFS and OS for salvage transplants with or without maintenance and describe the outcomes by types of maintenance utilized in this setting. Secondly, to redefine the optimal duration of remission post-first transplant in the maintenance era that would justify a second autologous transplant. Historically, in the pre-maintenance era, 24 months was demonstrated as an optimal remission post first transplant to gain benefit from a second salvage transplant.
Methods: This is a Canadian multicentre retrospective study utilizing the Canadian Myeloma Research Group Database, a national database with input from 16 Canadian centres hosting over 8700 patients. All patients included in the study had undergone a salvage ASCT between Jan 2012 to Dec 2021 at any line of treatment.
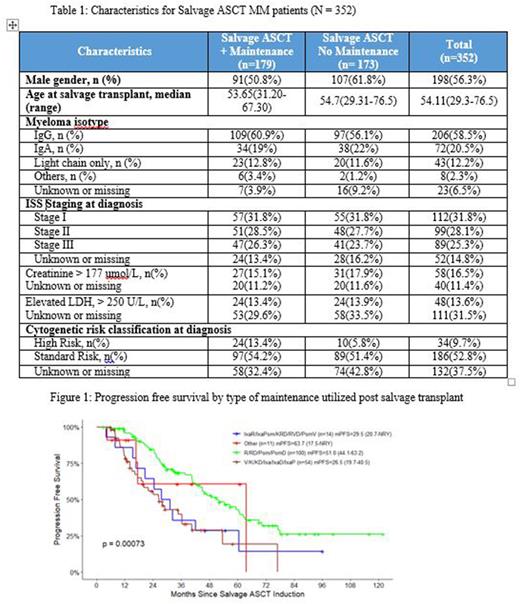
Results: Three hundred and fifty-two patients met eligibility for inclusion in this analysis. Baseline characteristics are portrayed in table 1. The median PFS (mPFS) for patients undergoing salvage transplant with (n= 179) and without (n=173) maintenance were 42.1 (34.8-53.6) and 24.2 (20.7-28.1) months respectively. The mOS was 101m (97.6-NYR) in the maintenance group and NYR (53.7-NYR) in the no maintenance group. In patients who received any type of maintenance post ASCT1 (n=169) and had a duration of response greater than 36m to the first transplant, the salvage transplant without maintenance (n=54) provided a mPFS of 17.3m (15.2-35). In a similar group that had greater than 36m response and received maintenance after ASCT1 (n=92), the addition of maintenance after the salvage transplants significantly improved the mPFS to 34.8m (26.5-51, p=<0.01). Patients that received maintenance post ASCT1 and had less than 36m PFS represent a higher risk group. In these patients, a salvage transplant without post salvage maintenance (n=10), yielded a mPFS of 9.9m (8.5-NYR). The addition of post salvage maintenance, however, significantly improved outcomes for this group as well (n=13) to a mPFS of 29.1(14.6-NYR). The most common type of maintenance post-salvage was imid based (55.9%), followed by PI based (30.2%) and then PI+imid (7.8%). Overall response rates for salvage transplants with or without maintenance therapy were 94.1% and 89.9% respectively and > VGPR were 75.3% and 67.6% respectively. The mPFS based on type of maintenance therapy are portrayed in figure 1. There was no statistically significant difference in OS based on types of maintenance therapy. Further analyses are pending and will be presented.
Conclusion: Salvage transplants followed by maintenance therapy in the first relapse space provide a meaningful duration of remission and this remains a good treatment option particularly in those with a long remission after their first ASCT. As novel immunotherapies such as CAR-T and bispecific antibodies move into earlier lines of treatment, this data could serve as an important real world benchmark when evaluating the landscape for these therapies.
Disclosures
Kaedbey:Beigene: Other: Advisory boards; BMS: Honoraria, Other: Advisory boards; Janssen: Honoraria, Other: Advisory boards; Sanofi: Other: Advisory boards; FORUS Therapeutics: Other: Advisory boards; Pfizer: Other: Advisory boards; Jewish General Hospital, Montreal, QC, Canada: Current Employment; Janssen, BMS, Sanofi, FORUS, Beigene, Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS. Janssen: Honoraria; BMS. Janssen: Honoraria; Janssen, BMS, Sanofi, FORUS, Beigene, Pfizer: Membership on an entity's Board of Directors or advisory committees. Hay:Kite/Gilead: Honoraria; Novartis: Honoraria; Bristol-Myers-Squibb: Honoraria; Jazz Pharmaceuticals: Honoraria; Janssen: Research Funding. McCurdy:Janssen: Honoraria; BMS: Honoraria, Research Funding; Sanofi: Honoraria; GSK: Honoraria; Forus: Honoraria; Amgen: Honoraria; Takeda: Honoraria. Chu:Gilead: Honoraria; Sanofi: Honoraria; Janssen: Honoraria; Bristol Myers Squibb: Honoraria. Jimenez-Zepeda:Pfizer: Honoraria; BMS: Honoraria; Amgen: Honoraria; GSK: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; FORUS Therapeutics: Honoraria; Janssen: Honoraria. Leblanc:FORUS Therapeutics: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Janssen: Honoraria; BMS: Honoraria. Song:Takeda: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria, Research Funding. Mian:BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria. Louzada:Pfizer: Honoraria; Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria. Sebag:BMS: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. White:GSK: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Forus: Consultancy, Honoraria; Antengene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria. Venner:BMS: Honoraria; Janssen: Honoraria; FORUS Therapeutics: Honoraria; Pfizer: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Takeda: Honoraria. Stakiw:Amgen: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria; BMS: Honoraria; FORUS Therapeutics: Honoraria; Janssen: Honoraria. Kotb:Akcea: Honoraria; Amgen: Honoraria; BMS: Honoraria; Janssen: Honoraria; Karyopharm: Current equity holder in private company; Merck: Honoraria, Research Funding; Pfizer: Honoraria; Sanofi: Honoraria, Research Funding; Takeda: Honoraria; Celgene: Honoraria. Reece:Sanofi: Honoraria; Karyopharm: Consultancy; Amgen: Consultancy, Honoraria; Millenium: Research Funding; BMS: Research Funding; Merck: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal